Answer:
0.16 Cal.
Step-by-step explanation:
What is given?
Specific heat of water (c) = 4.184 J/g °C.
Mass of water in can (m) = 48.6 g.
Change of temperature (ΔT) = 24.5 °C - 21.3 °C = 3.2 °C.
What do we need? The heat storedin Cheeto (Q).
Ste-by-step siolution:
To solve this problem, e have to use the following formula:
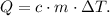
Where Q is heat, c is the specific heat, m is the mass and ΔT is the change of temperature.
We just have to replace the given values in the formula:
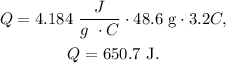
But we have to give the answer in units of Cal. Remember that 4.184 J equals 1 cal:
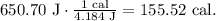
But we want to find Cal. Remember that 1 Calorie equals 1000 calories. The conversion will look like this:
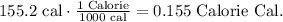
The answer is that te heat stored in the Cheeto is 0.156Cal.