ANSWER
4.15 kg
Step-by-step explanation
We have the volume and density and we want to find the mass. The formula for density is:
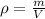
Solving for m:
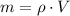
First we have to adjust the units. The density is given in g/mL and the volume is given in liters, so we can find the equivalent volume in mL:
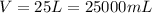
Now we replace and find the mass:
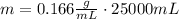
The mL gets cancelled and the result is in grams:
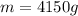
Which we have to divide by 1000 to find its equivalent in kilograms:
![m=4.15\operatorname{kg}]()