INFORMATION:
We know that:
- The atomic number of silicon (Si) is 14
And we must find its electron configuration
STEP BY STEP EXPLANATION:
To find it, we need to use the electron configuration chart.
In writing the electron configuration for Silicon, the first two electrons will go in the 1s orbital. Since 1s can only hold two electrons, the next 2 electrons for Silicon go in the 2s orbital. The next six electrons will go in the 2p orbital. The p orbital can hold up to six electrons. We'll put six in the 2p orbital, and then put the next two electrons in the 3s. Since the 3s if now full, we'll move to the 3p, where we'll place the remaining two electrons.
Then, the Silicon electron configuration would be
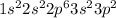
Now, using that
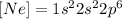
We can replace [Ne] in the electron configuration for silicon, and we finally obtain
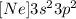
ANSWER:
A) [Ne] 3s2 3p2