Answer:
11.3 g of NH3 are produced. (The third option)
Step-by-step explanation:
1st) From the balanced equation we know that from 6 moles of H2O, 2 moles of NH3 are produced.
2nd) Using the molar mass of NH3 (17.031g/mol) we can convert moles to grams:
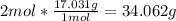
Now we know that when 6 moles of H2O react, 34.062g of NH3 are produced.
3rd) To calculate the grams of NH3 that will be produced from 2.00 moles of H2O, we can use a mathematical rule of three and the relation between H2O and NH3 that we found in the previous point:
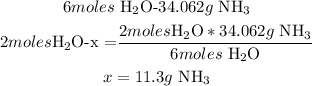
So, 11.3 grams of NH3 are produced.