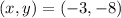So,
We're going to solve the system:
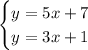
Using substitution.
What we're going to do, is to replace the expression for y in the second equation so we sould state a linear equation with only one variable:
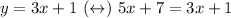
Now, let's solve this equation for x:
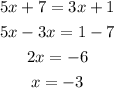
To find the value of y, we just replace the value of x in any of both equations:
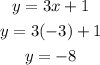
Then, the solution of the system is: