Answer
Step-by-step explanation
The given ions is:
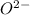
The number of electrons in a neutral oxygen atom is 8. if an oxygen atoms have a net charge of -2 like the ion given, it implies it has gained 2 additional electrons. Hence, the total number of electrons in O²⁻ ions = 8 + 2 = 10 electrons.
Therefore, using the orbital box diagram, an electron configuration for O²⁻ is: