ANSWER
OPTION B
Explanation:
Boyle's law state that "the volume of a given gas is inversely proportional to its pressure provided the temperature remains constant."
Mathematically, this law can be translated below as
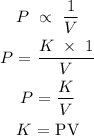
Therefore, when the temperature is constant, the temperature cancels out and it will remain only pressure and volume.
Hence, the correct option is option B