We could use the following equation for boiling point elevation:
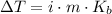
Where:
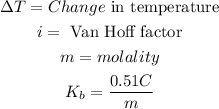
Now, remember that:
Since there are two elements in the compound dissociating, the Van Hoff's factor will be:
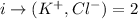
We just have to replace the given values and solve for the change in temperature:
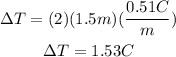
The change in temperature equals 1.53°C. As you know, the initial boiling point was 100°C, so, if temperature changed 1.53°C, the new boiling point of the solution is 101.53°C.