Answer:
q(x) = x^2 - 4x + 1
r(x) = 0
b(x) = x - 3
Step-by-step explanation:
Let us do the polynomial long division.
This tells us
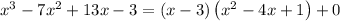
dividing both sides by x - 3 gives
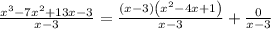
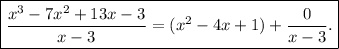
The above tells us that
q(x) = x^2 - 4x + 1
r(x) = 0
b(x) = x - 3.
which are our answers!