There are 6.13 moles of Ni in 360.0 grams.
1st) We need to know the atomic mass of Ni. We can find it in the Periodic Table of Elements.
Ni atomic mass: 58.69 g/mol
2nd) With a mathematical Rule of Three we can calculate the number of moles in 360.0 grams of Ni:
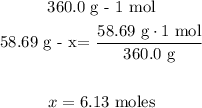
So, there are 6.13 moles in 360.0 grams of Ni.