Given data:
Mass of copper is,
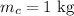
Initial temperature of copper is,
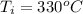
Final temperature of copper is,
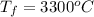
Melting point of copper is,
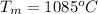
Specific heat capacity of copper is,
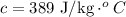
Latent heat of fusion of copper is,
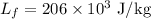
Boiling point of copper is,
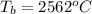
Latent heat of vaporization of copper is,
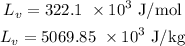
Now,
Formula of heat needed to change the temperature of copper,
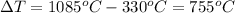
Formula:
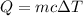
Substitute known values in above equation,
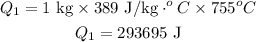
Formula for heat needed to melt copper is,
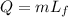
Substitute known values in above equation,
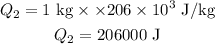
Formula of heat needed to raise temperature of copper,
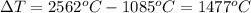
Formula:
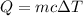
Substitute known values in above equation,
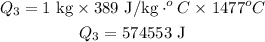
Now, formula of heat needed to vaporization of copper is as follows:
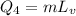
Substitiute known values in above equation,
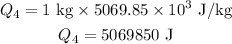
Formula of heat needed to change the temperature of copper,
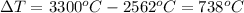
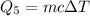
Substitute known values in above equation,
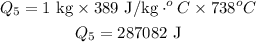
Above diagram is the phase diagram of copper.