Answer
To solve for the Absolute Zero, the Gay-Lussac's Law Temperature-Pressure Relationship in Gases is used.
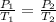
To find the absolute zero, prepare a graph of pressure vs. temperature in °C. From this graph you will get a straight line. Record the slope, y-intercept, and correlation coefficient (r). After that, use the equation for a line, y = mx + b that you would have got from the straight line. Absolute zero is the temperature at which the gas’s pressure equals zero. This is the line’s x-intercept. To calculate this value, set your y = 0, substitute in the value of the slope (m), and solve for x, from your equation. Your x will be the answer.
For the ideal gas law, the absolute zero can be extrapolated from volume vs temperature graph, the same way.