ANSWER
The coordinates of the midpoint are (-5, 2)
Step-by-step explanation
The midpoint is the point that's right in the middle of the segment. To find it's coordinates we have to find the middle between each coordinates of the endpoints.
The x-coordinate of the midpoint is:
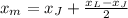
The second term of this expression is to find half the distance between the coordinates of the endpoints:
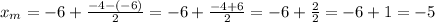
A similar expression is used to find the y-coordinate of the midpoint:
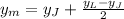
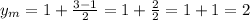
Therefore, the coordinates of the midpoint are (-5, 2)