So,
Remember that:
When the equilibrium constant K is too much bigger than 1, the equilibrium will shift to the products. In this case,
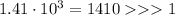
As the value of equilibrium constant is in multiple of thousand, the reaction will be more favourable in forward direction hence
Answer: The equilibrium reaction mixture will have a very high concentration of products.