Answer:
87.24 L.
Step-by-step explanation:
What is given?
Pressure = 1.03 atm.
Temperature = 22.36 °C + 273 = 295.36 K.
R = 0082 L *atm/mol*K.
Mass of NaN3 = 160.7 g.
Molar mass of NaN3 = 65 g/mol.
Step-by-step solution:
We have to find the number of moles of N2 to use the ideal gas law which has the formula:
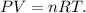
Where P is pressre, V is volume, n is the number ofmoles, R is the idelal gas constant and T is tthe hemperature on the Kelvin scale.
First, we have to convert 160.7 g of NaN3 to moles using its given molar mass, like this:
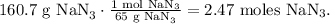
In the chemical equation, you can see that 2 moles of NaN3 reacted produces 3 moles of N2, so by doing a rule of three, we're going to find how many moles of N2 are being produced by 2.47 moles of NaN3:
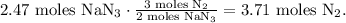
Now that we have the number of moles of N2, we can solve for 'V' in the initial formula and replace the given values that we have, like this:
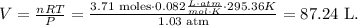
The answer i that hthe volume in liters of N2 gas is 87.24 L.