We have the next reaction
2C4 H10 + 13O2
And we must determine how many atoms of oxygen are needed on the product side.
To determine the number of atoms of oxygen that are need on the product side we need to use the law of the conservation of mass which says that matter cannot be created or destroyed. In chemical equations, the number of atoms of each element in the reactants must be the same as the number of atoms of each element in the products.
So, the number of atoms of oxygen that are need on the product side is the same number of atoms of oxygen on the reactants side.
Then, the number of atoms of oxygen on the reactants side is obtained multiplying the coefficient times the oxygen subscript
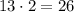
So, the are 26 atoms of oxygen in the reactants.
Finally, using the law of the conservation of mass 26 atoms of oxygen are needed on the pro