Answer: Al is the element that is being oxidized (oxidation number changed from 0 to +3) and H is the element reduced (oxidation number changed from +1 to 0)
Step-by-step explanation:
The question requires us to identify the species which are being oxidized and reduced in the following unbalanced chemical equation:
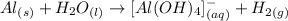
o identiyfy the species that are oxidizing and reducing in the reaction, we need to analye the oxidation number of the elements involved in the reaction. KSome important points to keep in mind are:
- the oxidation number of an element in simple compounds, such as Al and H2, is zero;
- the sum of oxidation numbers in an ion must be equal to the charge of the ion, while the sum of oxidation numbers in a neutal compound, must be 0.
Thus, we can say that the oxidation number in Al(s) is 0, and the oxidation number of H in H2 is also 0.
For H2O and [Al(OH)4]-, we can make the following analysis (the oxidation number of O is usually -2, while the charge of the ion (OH) is -1):
The oxidation numbers in the chemical equation can be summarized as:
- Al in Al(s): 0
- H in H2O: +1
- O in H2O: -2
- Al in [Al(OH)4]-: +3
- H in [Al(OH)4]- +1
- O in [Al(OH)4]-: -2
- H in H2: 0
Therefore, Al is the element that is being oxidized (oxidation number changed from 0 to +3) and H is the element reduced (oxidation number changed from +1 to 0).