Answer:
0.25 J/gºC
Explanations:
The formula for calculating the heat absorbed by the metal is expressed as:
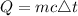
where;
m is the mass of the object
c is the specific heat capacity
△t is the change in temperature
Given the following parameters
m = 500g
△t = 78 - 25 = 53°C
Q = 6.64kJ = 6640Joules
Substitute
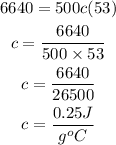
Therefore the specific heat capacity of this metal is 0.25 J/gºC