Answer:
1.598moles
Explanations:
According to the Avogadro's constant;
1 mole of an atom = 6.02 * 10^23 molecules
If there are 9.62 x 10^23 atoms of iron, the number of moles it contains is expressed as:
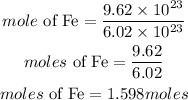
Hence the required moles of iron is 1.598moles