Answer:
17.37grams
Explanations:
Given the chemical reaction between ammonia and sulfuric acid expressed as:
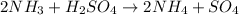
Given the following parameter
Mass of H2SO4 = 50 grams
Determine the moles of sulfuric acid
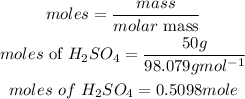
According to stoichiometry, 2moles of ammonia reacted with 1 mole of sulfuric acid, hence the number of moles of ammonia needed is given as:
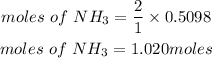
Determine the required mass of NH3
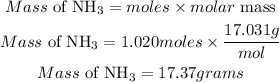
Therefore the mass of ammonia (NH3) needed to completely react with 50 grams of sulfuric acid is 17.37grams