Let x be the volume of the 14% HCl solution and y the volume of the 19% HCl solution, in mililiters.
Since the total volume (in mililiters) of the mix should be 330, then:
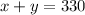
The total amount of HCl on the first mix is (14/100), on the second mix is (19/100)y and on the final mix, is (17/100)(330). Then:
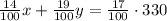
Isolate x from the first equation and substitute the resulting expression into the second one. Then, solve for y and go back to the first equation with the value of y to find the value of x:
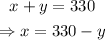
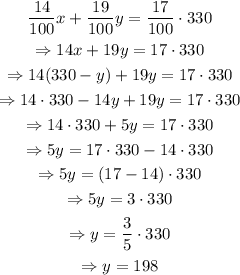
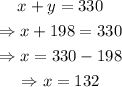
Therefore, 132 ml of 14% HCl solution and 198 ml of 19% HCl solution are needed to create 330 ml of 17% HCl solution.