Given that the temperature , T = 10 deg C and mass of the gas is, m = 125 g.
The specific heat is given as S = 4.184 J/g deg C
To find the heat required, Q.
The formula to find heat required is
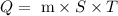
Substituting the values in the above formula, we get
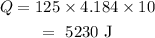
Thus, 5230 J heat is required to raise the temperature by 10 deg C.