We have as a reagent a salt, lead nitrate (Pb(NO3)2), and an unknown solution that gives us as a product lead chloride (PbCl2). That is, the solution must contain chlorine.
If a chlorine solution is used we will have the following reaction:
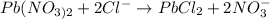
So, with a chlorine solution, we will have a white precipitate of lead chloride.