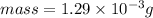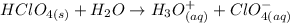
B) To determine the concentration from pH we use the following formula:
![\begin{gathered} pH=-log[H_3O^+] \\ 1.28=-log[H_3O^+] \\ [H_3O^+]=10^(-pH) \\ [H_3O^+]=10^(-1.28) \\ [H_3O^+]=0.052\text{ }molL^(-1) \end{gathered}](https://img.qammunity.org/2023/formulas/chemistry/college/fyki0km0a27e6ceeu543p8uy3eou3pa23z.png)
C) Determining the mass of perchloric acid used to prepare the solution, we must first determine the number of moles in the solution.
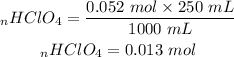
Mass is therefore:
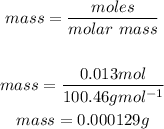
Mass prepared is 1.29e-3 g.