Answer:
Step-by-step explanation:
Here, we want to get the names or formulas of the given hydrates
2) Copper (I) Sulfate pentahydrate
Penta means 5 and pentahydrate means there are 5 molecules of water of crystallization
We have the Copper (I) Sulfate as:
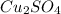
With the pentahydrate, we have it as:
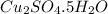
7) As we can see here, the root compound is Sodium Chromate
The water of crstallization is 4 molecules
The name of the compound is thus:
Sodium Chromate tetrahydrate