Step 1 - Understanding the reaction between Calcium and EDTA
EDTA is a molecule that always reacts in an equimolar fashion with any metal. Therefore, we always need one mole of EDTA for one mole of the metal.
We can represent its reaction with Ca(2+) in a simplified manner as:
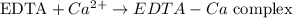
Since the reaction is equimolar, the quantity spent in moles of EDTA is exactly the same as the quantity of moles of Ca(2+) in the tap water sample.
Step 2 - How can we calculate the concentration of Ca(2+) in tap water?
Once we know the amount of EDTA present when the color change occurs,
we can determine the concentration of calcium present in the tap water by
converting the volume of EDTA to...?
Well, first of all, let's remember the definition of concentration:
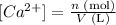
Therefore, to obtain the concentration of Ca(2+) in tap water, we just need to divide the number of moles of Ca(2+) in the sample by its volume.
Since the number of moles of Calcium can be obtained directly by the number of moles of EDTA (see step 1), the correct way to proceed is converting the volume of EDTA to moles and then take that number and divide it by the initial volume of tap water in liters.
The correct alternative is thus item b).