Answer
The molarity of iodine in the solution = 4.7122 mol/L
Step-by-step explanation
Given:
Mass of iodine = 213.95 g
Volume of the solution = 357.79 mL
What to find:
The molarity of iodine in the solution.
Step-by-step solution:
The molarity of iodine in the solution can be calculated using the molarity formula below.
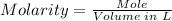
Moles of iodine in 213.95 g iodine can be known using the mole formula.
The molar mass of iodine = 126.90 g/mol
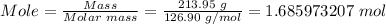
Also, volume in L = (357.79mL/1000 mL) x 1 L = 0.35779 L
Putting the values of mole and volume in L into the molarity formula above, we have;
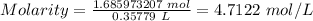
Therefore, the molarity of iodine in the solution is 4.7122 mol/L