Alcohol solution
You need 525 ml of a 85% alcohol solution. Then, the percentage of pure alcohol you need is found by multiplying 525 ml by 0.85:
525 ml · 0.85 = 446.25 ml of alcohol
And the percentage of the solution of another substance different to alcohol, let's say water, is 15%. Then you will need
525 ml · 0.15 = 78.75 ml of water
Mixture
If we have on hand a 25% alcohol mixture, then the other 75% corresponds to another substance, let's say water. That is
our mixture has 25% of alcohol and 75% of water.
The whole 15% of water of your solution (= 78.75 ml) will be obtained by the 75% water of this mixture.
We want to find the whole quatity of the mixture we need.
Since 75% corresponds to 78.75 ml, we want to find how much mixture corresponds to the 100%. Then we have the following equivalence:
100% → ??
75% → 78.75 ml
If we divide both sides of the equivalence we will obtain the same result:
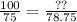
Multiplying by 78.75 ml both sides:
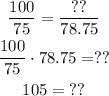
Then the 100% of the mixture we need is 105 ml
Pure alcohol
Now, let's find the alcohol we need.
We have that 25% of 105 ml is alcohol. That is
105 ml · 0.25 = 26.25 ml of alcohol
The total quantity of alcohol we need (as we found at the beggining ) is 85% of 525ml:
525 ml · 0.85 = 446.25 ml
Due to the mixture we have 26.25 ml. Then the quatity of pure alcohol missing is
446.25 ml - 26.25 ml = 420 ml