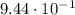We know that 0.0350 moles of aluminum participated in a chemical reaction and we must find the mass of the aluminum that participated in the reaction.
In order to find the mass of the aluminum that participated we must use the molar mass of this element:
- Molar mass of Aluminum: 26.981539 g/mol
Then, we need to use the next formula:
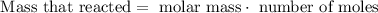
In this case, the number of moles is 0.0350
Now, we must replace the values in the formula
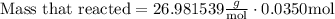
Finally, we must simplify the equation
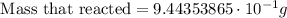
ANSWER:
Using the correct number of significant digits: