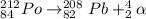Polonium-212 undergoes alpha decay to produce lead-208, which means that it gives off alpha radiation, i.e. alpha particles.
An alpha particle, α, is simply the nucleus of a helium-4 atom, which means that it contains 2 protons and two neutrons.
When a radioactive nuclide emits an alpha particle its mass number will decrease by 4, and its atomic number will decrease by 2.
Then, the balanced nuclear equation for this process looks like this: