Given:
x+y=5
x-y=-1
To solve the system of equations graphically, we note first that if the lines intersect, the point of intersection is the solution to the system. The graph of the lines are shown below:
As we can see, the lines intersect at point (2,3). Hence, the solution is:
x=2
y=3
To check the solution, we plug in x=2 and y=3 into the given equations:
For x+y=5:
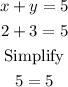
For x-y=-1:
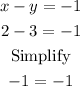
Therefore, the solution is correct.