Step 1
Charles' law states that the volume occupied by a fixed amount of gas is directly proportional to its absolute temperature if the pressure remains constant.
Mathematically:
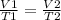
----------------
Step 2
Information provided:
V1 = volume 1 = 10.0 L
T1 = temperature 1 = 298 K
V2 = volume 2 = 15.0 L
T2 = temperature 2 = unknown
--------------
Step 3
Procedure:
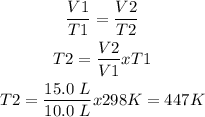
Answer: T2 = 447 K