We have to do a dimensional analysis. We have to convert from 25.9 grams of Cr2O3 to moles using the molar mass of Cr2O3 which is 152 g/mol (You can find the molar mass using the periodic table). Then, we have that in 1 mol of Cr2O3, there are 2 moles of chromium (Cr) and finally, the value that we obtain is converted to grams of Cr using the molar mass of Cr which is 52 g/mol.
This dimensional analysis would look like this:
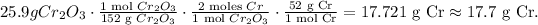
The answer is that the mass of chromium is 17.7 grams.