Answer
The average atomic mass of Rb = 85.6 amu
Step-by-step explanation
Abundance of ⁸⁵Rb = 72.2% = 0.722
Atomic mass of ⁸⁵Rb = 85
Abundance of ⁸⁷Rb = 27.8% = 0.278
Atomic mass of ⁸⁷Rb = 87
Average atomic mass = ?
First, the exact atomic mass of each isotope must be obtained. Then multiply the percent abundance as a decimal for both isotopes and add them together i.e
Average atomic mass of Rb = (abundance of ⁸⁵Rb × its atomic mass) +(abundance of ⁸⁷Rb × its atomic mass) / 100
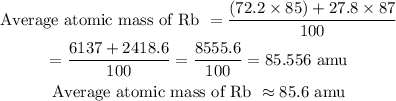
The average atomic mass of rubidium is 85.6 amu