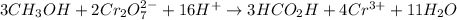Answer:
![3CH_3OH+2Cr_2O_7^(2-)+16H^+\operatorname{\rightarrow}3HCO_2H+4Cr^(3+)+11H_2O]()
Expanations:
Given the unbalanced chemical reaction
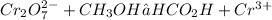
Separate into half reactions
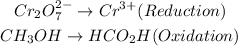
Balance all other atoms except hydrogen and oxygen to have:
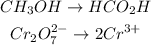
Balance the oxygen atoms by adding water
![\begin{gathered} CH_3OH+H_2O\rightarrow HCO_2H \\ Cr_2O_7^(2-)\operatorname{\rightarrow}2Cr^(3+)+7H_2O \end{gathered}]()
Balance the hydrogen atoms by adding hydrogen ion
![\begin{gathered} CH_3OH+H_2O\operatorname{\rightarrow}HCO_2H+4H^+ \\ Cr_2O_7^(2-)+14H^+\operatorname{\rightarrow}2Cr^(3+)+7H_2O \end{gathered}]()
Balance the charges
![\begin{gathered} CH_3OH+H_2O\operatorname{\rightarrow}HCO_2H+4H^++4e^-\text{ * 3} \\ Cr_2O_7^(2-)+14H^++6e^-\operatorname{\rightarrow}2Cr^(3+)+7H_2O\text{ * 2} \\ _(------------------------------------) \\ 3CH_3OH+3H_2O\operatorname{\rightarrow}3HCO_2H+12H^++12e^- \\ 2Cr_2O_7^(2-)+28H^++12e^-\operatorname{\rightarrow}4Cr^(3+)+14H_2O \end{gathered}]()
Cancel the charges and add the resulting equation to have: