The pH tells us how acidic or basic a substance is. Its value depends on the concentration of hydrogen ions, the pH equation is defined as:
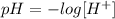
Where,
H+ is the concentration of hydrogen ions
So to find the pH we must substitute the concentration of H+ ions into the equation:
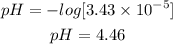
Answer: The pH of the solution will be 4.5