Given:
Mass = 1.6 kg
Temperature = 20°C
Let's determine how much heat must be removed from the water in order for all of it to freeze.
Apply the formula:
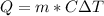
The freezing point of water is 0°C
Where:
m is the mass = 1.6 kg
C is specific heat of water = 4200 J/kg°C
T is the temperature change
Thus, we have:
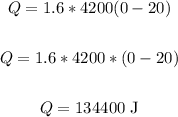
Therefore, the amount of heat lost is 134400 J.
ANSWER:
134400 J