Answer:
0.4613 atm
Explanations:
To convert 350.8torr to atm, we will use the conversion rate.
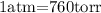
We are to convert 350.8 torr to atm, we can express this as:
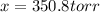
x is the pressure in units of atm
Divide both equations to have:
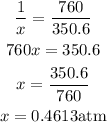
Hence the pressure of the gas in units of atm is 0.4613 atm