Answer:
k = 1.7 x 10^-3
Explanations:
Given the the reaction between nitrogen and oxygen gas expressed as:
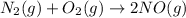
The equilibrium constant for the reaction is expressed as:
![k=([NO]^2)/([N_2[O_2])](https://img.qammunity.org/2023/formulas/chemistry/college/elixnuqw0bo5bhcrxbkbxjo5qq3492wli1.png)
Given the following concentrations
[NO] = 0.034M
[N2] = 0.69
[O2] = 0.98
Substitute the concentrations into the formula to have:
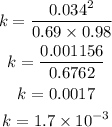
Hence the equilibrium constant for the reaction is 1.7 x 10^-3