Answer: the pair magnesium chloride and silver nitrate (letter A) would result in a precipitate when mixed
Step-by-step explanation:
The question requires us to determine which mixture among the options given , would result in a precipitate.
To olve this problem, we'll need to analyze the chemcical reacton that happens when the mixtures o f solutions are made.
Note that most of the reactions between the soluions wgiven ill ahappen through a double displacement mechanism, which can be written as:
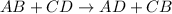
A) mixing magnesium chloride (MgCl2) and silver nitrate (AgNO3) would produce silver choride (AgCl) and magnesium nitrate (Mg(NO3)2):
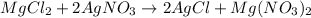
B) Mixing sodium sulfate (Na2SO4) and magnesium nitrate (Mg(NO3)2) produces sodium nitrate (NaNO3) and magnesium sulfate (MgSO4):
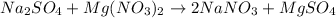
C) Mixing sodium chloride (NaCl and opotassium nitrate (KNO3) produces sodium nitrate (NaNO3) and potassium chloride (KCl):
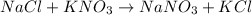
D) Mixing sodium hydroxide (NaOH) and nitric acid (HNO3) results in the formation of the salt odium nitrate (NaNO3) and water (H2O):
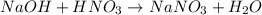
Among the list of possible products obtained (AgCl, Mg(NO3)2, NaNO3, MgSO4, KCl and H2O), only silver chloride presents low solubility in water, thus it would form a precipitate in aqueous medium.
Therefore, the pair magnesium chloride and silver nitrate (letter A) would result in a precipitate when mixed.