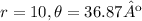Problem
Find the polar coordinates for the point (-8,6)..
Solution
For this case we need to find the radius given by:
![r=\sqrt[]{(-8)^2+(6)^2}=10](https://img.qammunity.org/2023/formulas/mathematics/college/owpg59sf7izgnpsmfs75umd9icap8j06m4.png)
Now we can find the cosine of the angle on this way:
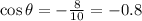
And the sine would be:
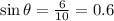
We can find the angle with this operation:
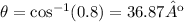
And then our answer would be: