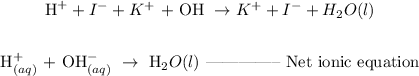ANSWER
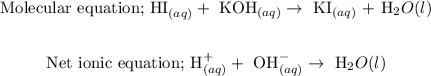
Step-by-step explanation
Part A
Step 1: Write the molecular formula for both hydroiodic acid and potassium hydroxide
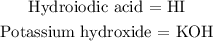
Step 2: Write a balanced equation between the two compounds
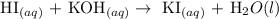
Hence, the molecular equation for the reaction is
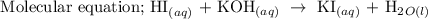
Part B
Write the ionic equation of the reaction in part A
To write the ionic equation of the above equation, you need to understand certain rules listed below
1. Only compounds that the exit in the aqueous state can be written in the ionic form
2. Compounds that exist in the liquid, solid, and gaseous phase can't be broken into ionic form
Step 1: write the molecular equation in the ionic form
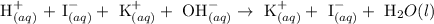
Step 2: Cancel the spectator ions
Spectator ions are those ions that are present on both sides of the equation