Hello
The question here is a chemical reaction that requires us to balance
The reaction between potassium and hydrochloric acid
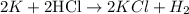
To identify the oxidizing and reducing agent here, i would like to bring a defination about oxidation and reduction in terms of hydrogen transfer
Oxidation: This is defined as the process of loss of hydrogen atom during a chemical reaction.
Reduction: This is the process of gain of hydrogen atom during a chemical reaction.
So from the chemical reaction, the oxidized element i.e element that lost hydrogen atom is HCL while the reduced element i.e the element that gains hydrogen atom is K.
NB: The reduced element becomes the oxidizing agent (element that brings about oxidation) and the oxidized element becomes the reducing agent (element that brings about reduction)
Oxidation half equation
![undefined]()