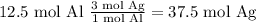Answer
37.5 mol Ag
Procedure
The first step for solving this problem will be searching for the chemical reaction that occurs between silver nitrate and aluminum.
Al + AgNO₃ = Ag + Al(NO₃)₃
Then you will want to get the balanced equation by trial and error method.
Al + 3AgNO₃ = 3Ag + Al(NO₃)₃
Now use the stoichiometry of the reaction to determine the moles, assuming the silver nitrate is the excess reagent.