Answer;
Theoretical yield = 8.33 grams
percent yield = 21.7%
Explanations:
Given the balanced chemical reaction between hydrogen and nitrogen to produce ammonia expressed as:
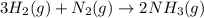
Given the following parameters
Mass of H2 = 1.48grams
Mass of N2 = 9.97grams
Determine the moles of each reactant
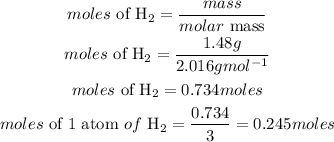
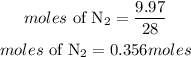
Since the moles of 1 atom of H2 gas is lower than that of nitrogen gas, hence H2 is the limiting reactant
According to stoichiometry, 3 moles of H2 produces 2 moles of ammonia, the moles of ammonia required will be:
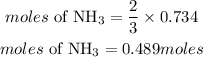
Determine the mass of NH3 (theoretical yield)
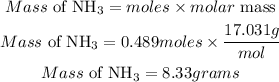
Hence the theoretical yield in grams for this reaction is 8.33 grams
Determine the percentage yield

Therefore the percent yield for this reaction is 21.7%