The first step to answer this question is to use the molar mass of HNO3 to convert the given grams of this substance to moles (MM=63.0g/mol):
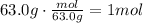
Now, divide the moles of solute by the kg of solvent to find the molality of the solution:
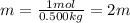
The molality of the solution is 2m.