Answer:
Step-by-step explanation:
Here, we want to write the balanced precipitation reactions:
1) Calcium Nitrate and Potassium Iodide
There is no reaction between these two. Hence, we do not write a chemical equation for it. This can be seen directly from the table as indicated by NR, which means no reaction.
2) Calcium Nitrate and Potassium Hydroxide
We have the equation of the reaction as follows:
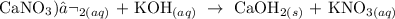
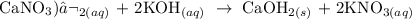
The precipitate formed here is calcium hydroxide, which is the faint white precipitate
3) Calcium Nitrate and Sodium oxalate
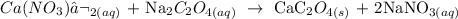
Calcium oxalate is the precipitate here, which is the white precipitate
4) Calcium Nitrate and Sodium Sulfate:
There is no reaction here. This can be seen indicated by NR