ANSWER
The pressure of the gas is 0.4376 atm
Step-by-step explanation
Given information
The number of moles of CO2 = 4 moles
The temperature = 80K
The volume of the gas = 60L
To find the pressure of the gas, follow the steps below
Step 1: Assume the gas behaves like an ideal gas
Step 2: Write the ideal gas equation
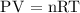
Where
V = 60L
n = 4 moles
T = 80K
Step 3: Substitute the given data into the formula in step 1

Hence, the pressure of the gas is 0.4376 atm