From the equation of reaction, 2 moles of O₂ reacted to produce N₂O₄.
Using moles-mass-molar mass relationship
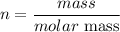
Let's find the mass of O₂ in the equation of reaction.
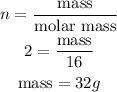
Mass of N₂O₄ in the equation of reaction.
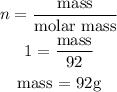
from the calculation above, 32g of O₂ produce 92g of N₂O₄
x g of O₂ will produce 47.6g of N₂O₄
cross multiply both sides and make x the subject of formula.
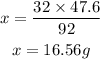
From the calculations above, 16.56g of O₂ will produce 47.6g of N₂O₄.