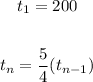You use the next formula to get a explicit equation for a geometric sequence:
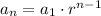
In this case you use as the first data 2000 (term when n=1).
r is the common ratio between each term.
To find r you divide each term into the previous term as follow:
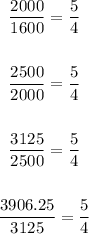
Then, you get the next explicit equation: writen in two different forms
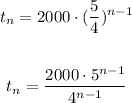
------------------------------------------
For the recursive formula you have the next:
Where tn-1 is the previus term
For the given sequence: